Combined optical single molecule and atomic force microscopy to elucidate enzyme-induced collagen degradation kinetics
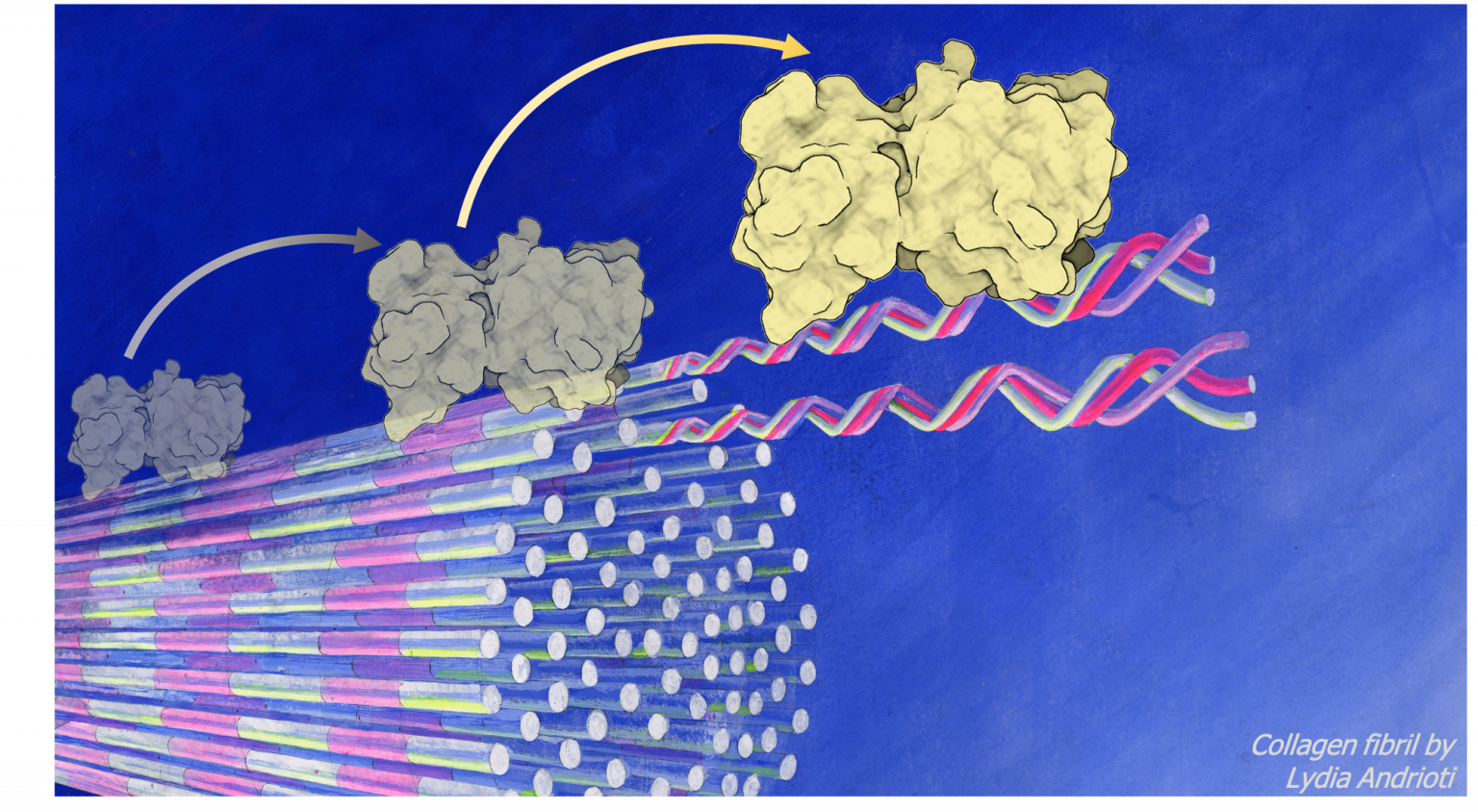
Artist’s impression of spatiotemporal digestion kinetics of a collagen fibril by a matrix metalloproteinase
Enzymatic digestion of collagen fibrils is vital to wound healing and preventing scar tissue formation that otherwise leads to fibrosis in pathologies such as liver cirrhosis or idiopathic pulmonary fibrosis. When collagen fibril digestion is impaired, accumulation of collagen fibrils takes over, disrupting collagen turn over, increased cross-linking and eventually impaired tissue mechanics, structure and organ functionality. Although spatiotemporal digestion kinetics have been explored, specific mechanisms of digestion kinetics are farm from being understood. How is matrix metalloproteinase (MMP) activity on mechanically loaded collagen fibrils affected or in damaged? Is pathological cross-linking in collagen fibrils hindering such degradation and, if yes, how?
Matrix metalloproteinases (MMPs) degrade collagen fibrils of the extra-cellular matrix (ECM), and play a pivotal role in patho-physiological processes by affecting mechanical stiffness and toughness of tissues and organs. On the one hand, degradation makes space for new collagen formation during physiological repair and adaptation during wound healing or tissue remodelling. On the other hand, it is an important step in pathology progression such as in fibrosis1, cancer2 or osteoarthritis3.
Collagen fibrils form through a self-assembly process of ultra-high aspect ratio collagen molecules . The nature of collagen molecules and their self-assembly results to collagen fibrils possessing their unique ultra-structure and composition governed and sustained by strong intermolecular interactions. Intermolecular interactions are both non-covalent (hydrogen bonds, van der Waals etc.) and covalent (enzymatic and non-enzymatic cross-links). Eventually the ECM is build mainly from collagen fibrils which possess high extensibility and strength. At the sub-micrometer, collagen fibrils are the main structural component of the cell microenvironment, the physical properties of which play important roles and are affecting/stimulating cell responses.Collagen fibrils, although similar at first sight (as seen through AFM imaging by identifying their D-periodicity), their composition and inter-molecular chemistry differs among tissues in the body. Interestingly, collagen fibrils from different tissues, show varying remodelling rates and dysregulation of MMPs play a central role in development and progression of pathologies such as fibrosis. To better understand what causes changes in the digestion of collagen fibrils and why digestion is impaired in fibrotic tissues, we have to take a closer look at the (un-)binding kinetics and the spatiotemporal changes in structure and mechanics at the length scale level of collagen fibrils.
Structure-function relationships of the enzymatic digestion at the collagen fibril level can be determined by combining imaging modalities that provide access to information on the kinetics of MMP (un-)binding and the mechanical/structural properties of the collagen fibril. In our project we combine atomic force microscopy (AFM) and single molecule fluorescence microscopy (SMFM) to study the spatiotemporal mechanisms of collagen degradation by matrix metalloproteinases. We hence use AFM – as the perfect tool for imaging mechanics and structures at nanometer resolution – together with SMFM – as the ideal method or quantifying binding kinetics. Combining the two modalities in the same instrument for simultaneous operation enables for the first time the direct correlation between MMP binding with the consequence on collagen structure and mechanics at the degradation locus.
Research group
Principal investigator:
Orestis G. Andriotis
Biomechanics Research Unit
Institute of Lightweight Design and Structural Biomechanics, TU Wien, Austria
orestis.andriotis@tuwien.ac.at
Co-Principal investigators:
Gerhard Schütz
Biophysics GroupInstitute of Applied Physics, TU Wien, Austria
schuetz@iap.tuwien.ac.at
Philipp J. Thurner
Biomechanics Research Unit
Institute of Lightweight Design and Structural Biomechanics, TU Wien, Austria
philipp.thurner@tuwien.ac.at
Doctoral students
Manuel Rufin
Biomechanics Research Unit
Institute of Lightweight Design and Structural Biomechanics, TU Wien, Austria
rufman@ilsb.tuwien.ac.at
Simon Jaritz
Biophysics Group
Institute of Applied Physics, TU Wien, Austria
jaritz@iap.tuwien.ac.at
Martin Fölser
Biophysics Group
Institute of Applied Physics, TU Wien, Austria